Raphaël Ceccaldi When DNA repair becomes the Achilles heel of tumours
Inserm researcher and head of the “Alternative DNA repair mechanisms in human cancers” team at Institut Curie, Paris
- 2025 • Impulscience

DNA breaks, which pose a threat to genome stability, are constantly and reliably repaired by the cell. One of these repair mechanisms has been found to be an ally of certain cancer cells. Raphaël Ceccaldi and his team are seeking to learn everything there is to know about DNA break repair in these cells in order to improve therapeutic strategies for cancer treatment.
Breaks that threaten genome stability
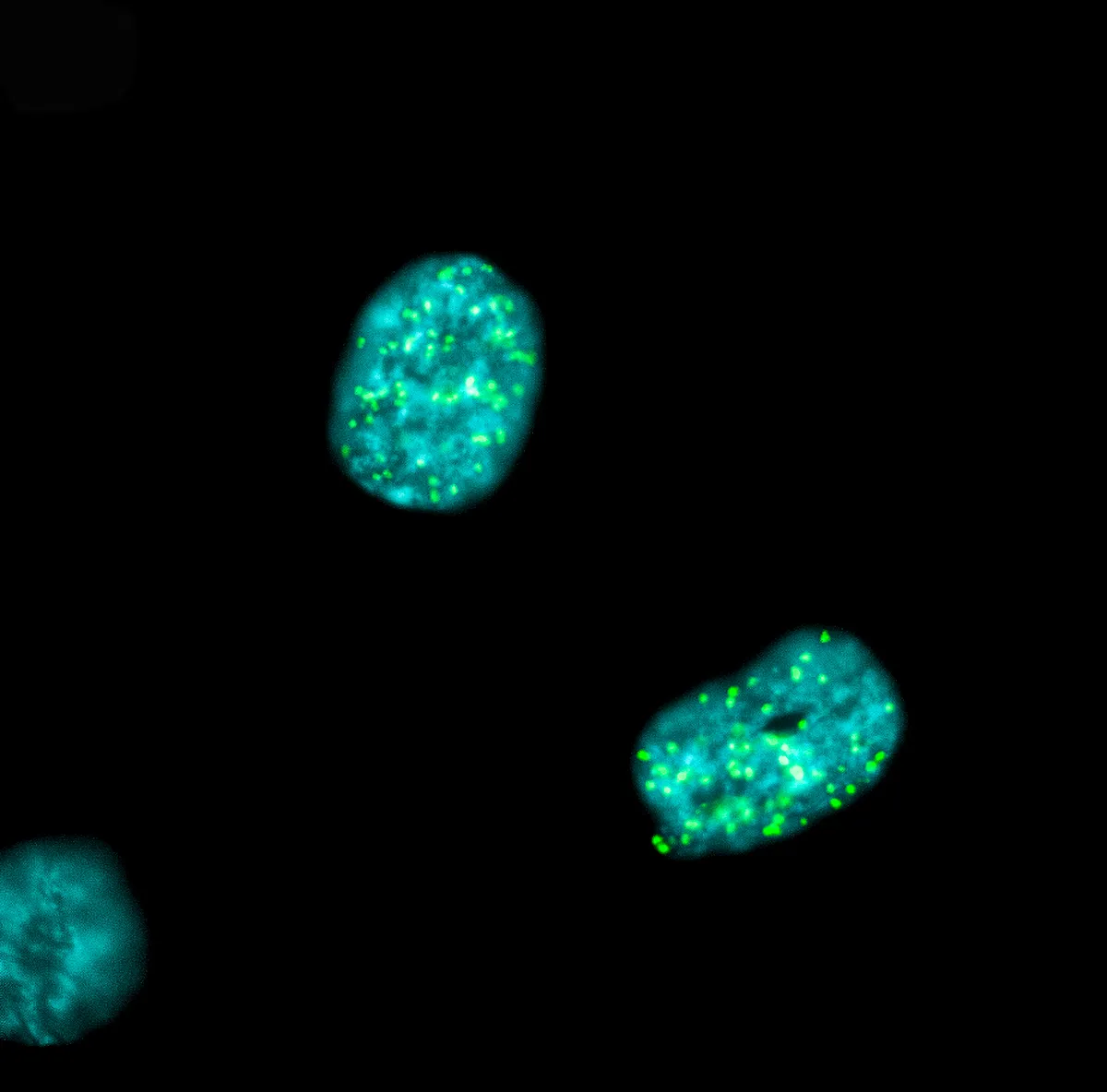
The DNA in our cells is regularly damaged. Breaks affecting both strands of the double helix are among the most toxic. These breaks can be caused by external sources (exposure to radiation, chemical molecules, etc.) or internal sources (free radicals, errors during DNA replication). If they are not repaired, they threaten the stability of the genome, potentially leading to errors in the genetic code with serious consequences, such as the development of cancer.
The repair of these breaks depends on the activity of multiple DNA repair mechanisms throughout the cell cycle, the process that allows cells to divide. For example, during the phase of the cycle in which the cell grows, doubles its genetic material and prepares to divide into two daughter cells, DNA breaks are mainly repaired by mechanisms called “non-homologous junction” and “homologous recombination”.
These two mechanisms are completely inhibited during mitosis, the next phase of the cell cycle where genetic material is shared between the two daughter cells, in order to avoid serious problems during sharing. However, DNA repair during mitosis is not completely absent.
A repair mechanism essential for the survival of cancer cells
In 2023, Raphaël Ceccaldi's team discovered that tumour cell survival depends on the repair of DNA breaks during mitosis. This is particularly true in types of cancer that do not have a “homologous recombination” repair mechanism, which is frequently observed in breast and ovarian cancer. Their work identified DNA polymerase theta (Polθ) as one of the key factors involved in this repair process during mitosis. Although its role is essential for the survival of cancer cells, the precise mechanisms of this recently discovered pathway remain unclear.
The life cycle of DNA breaks and therapeutic approaches to cancer
Using a multidisciplinary approach, Raphaël Ceccaldi and his team aim to study the molecular details that govern DNA breaks that occur during mitosis: their formation, repair, and transmission to daughter cells. Impulscience will support Raphaël Ceccaldi's team by enabling it to expand and to acquire a high-resolution microscope. The team will thus be able to develop an ambitious project, aiming to understand how genome stability is maintained during mitosis. To do this, it will explore the pathways that prevent the formation of DNA breaks and study the consequences of the transmission of unrepaired breaks to daughter cells.
Ultimately, this project will enable Raphaël Ceccaldi's team to identify and test therapeutic targets that are likely to act at different stages of the mitotic DNA breakage life cycle, in order to improve and to better target cancer therapies.
Raphaël Ceccaldi in a few words
After two PhDs, one in pharmacy and one in science, Raphaël Ceccaldi pursued his research on cancer during a postdoctoral fellowship at the Dana-Farber Cancer Institute, Harvard Medical School (United States). In 2018, he set up his research team at the Institut Curie, dedicated to studying the DNA repair mechanisms essential for the survival of cancer cells. Raphaël Ceccaldi and his team aim to dissect these molecular pathways in order to identify new therapeutic targets and contribute to the development of innovative cancer treatments.